Approval test for therapy splint
Problem: Mechanical tests on a PMMA occlusal splint
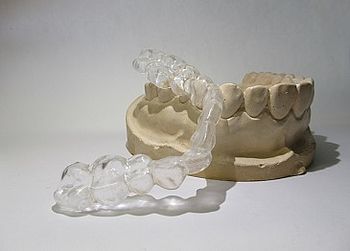
For the sale of therapeutic splints (Fig. 1) on the American market, medical products are subject to approval by the Food and Drug Administration, FDA.
The splints are made of a PMMA material. The greatest danger with this product is that fractures and splinters can occur during sleep due to grinding or improper tilting, which can lead to suffocation. Such environmental conditions of typically 37 °C at high humidity must be taken into account in the test set-up for mechanical tests.
In order to obtain approval, the international standard ISO 20795-1 “Dentistry on denture resins” requires, among other things, mechanical tests to be carried out. Another ISO requirement is direct comparison with a product already approved on the market.
Method: Determination of material properties according to ISO 20795-1
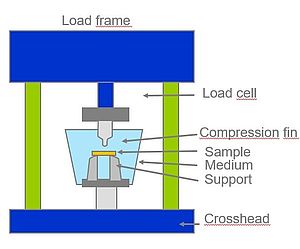
After the preparation of suitable test specimens by means of grinding and milling, the flexural properties are tested in a tempered, aqueous environment (Fig. 2).
The determined properties of the new therapy splint are compared with the properties determined on the competitor's product.
Result: Evaluation of the flexural properties
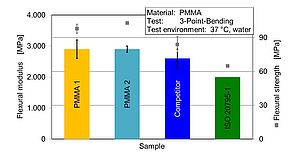
Based on the bending stress/strain diagrams determined in the bending test, the characteristic values of the flexural modulus and the flexural strength in water at 37 °C can be derived. Figure 3 shows a good agreement between two different PMMA therapy splint batches as well as a surpassing of the determined properties in comparison to the competitive product.
Considering the standard specification of Efnominal = 2000 MPa for the flexural modulus, all the therapy splints tested can exceed the required stiffness. With regard to the flexural strength, the behaviour is comparable. The two PMMA materials can be considered equal with a flexural strength of σf = 100 MPa. Compared to the competitor product with σf ≈ 80 MPa and the standard specification of σfnominal ≥ 65 MPa, these are clearly exceeded (Figure 3).
The comparison with standard specifications and already approved products enables a reliable assessment of the mechanical properties of the "new" therapy splint.
Any questions?